Epilepsy Control
A newly discovered link between order in the activity of neurons in the brain and excitability—how likely it is that individual neurons will “fire”—may provide a means for monitoring treatment of conditions like epilepsy that would be less invasive and thus more versatile than current methods. This new approach, developed by NIMH scientists, has implications beyond conditions like epilepsy; the findings support an emerging picture of how the brain balances flexibility and order during wakefulness and sleep.
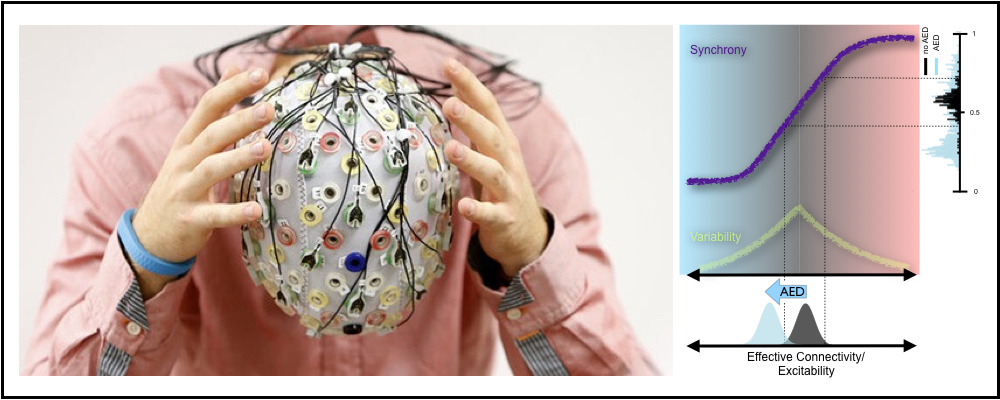
The brain is a network of billions of neurons that “excite” each other. These interactions must be balanced across the brain to allow brain networks to function properly without tipping into chaos. NIMH researchers Christian Meisel and Dietmar Plenz led a collaboration of scientists from the USA, Germany, Australia, and Switzerland to investigate how the brain achieves this balance by tracking the level of order in neuronal activity. Previous studies using computers and cell culture had suggested that the degree of order is closely related to the degree of excitability. When excitability is low, network activity is disordered; as excitability grows, a point is reached where order abruptly emerges, reminiscent of a phase transition like that which occurs as water turns to ice.
In this work, the scientists identified a close relation between network order and network excitability in the human brain. They analyzed the level of order in brain activity recorded over many days in patients suffering from epilepsy and showed that measures of spontaneous order track with conventional measures of excitability that use electrical or magnetic stimulation. Importantly, they showed that in patients taken off antiepileptic medication, brain networks were at the threshold between disorder and order: exactly what earlier work predicted would be true for the living brain. In contrast, brain networks became less ordered, when the dosage of antiepileptic medication was increased. (Antiepileptic medication reduces excitability.)
In humans suffering from epilepsy, seizures result from abnormal changes in neuronal excitability; the reduction of excitability with antiepileptic drugs is therefore of prime clinical importance. “By simply monitoring spontaneous brain activity, the amount of order in the brain can be quantified, providing information about the underlying excitability of brain networks,” said NIMH scientist Christian Meisel. Up to now, reliable measures of brain excitability had been difficult to obtain. In the clinical environment, this new approach might be particularly useful because traditional measures of excitability relied on active perturbation of the brain which often cannot be used in epilepsy patients and over longer periods of time.
Taking advantage of the new approach for long-term monitoring, the researchers demonstrated that order and—consequently excitability—in the brain increases during the day and decreases at night. Thus, sleep seems to be important in rebalancing excitability in the brain. Such versatile monitoring of excitability levels in patients could guide more individualized treatment strategies.
C. Meisel
C. Meisel, A. Schulze-Bonhage, D. Freestone, M. Cook, P. Achermann, D. Plenz
The Function of Sleep
Sleep is essential for daytime functioning and well being. Without sleep optimal brain functioning such as responsiveness to stimuli, information processing, or learning is impaired. The neuronal correlates underlying the decline in cognitive performance, however, have been difficult to identify. This shortcoming may be a result of an insufficient understanding of what determines and constitutes even normal cortical functioning and information processing. Although a generally accepted framework of how brain networks process information in optimal ways is still missing, there has been considerable success in approaching some of the brain’s functions from a physics perspective. The relevance of such a dynamical system framework for brain functioning goes back to the 1950s when Alan Turing suggested it as a way the nervous system is afforded the speed and flexibility required for instantaneous reaction to novelty. Since this time it has been a recurrent idea that brain networks might operate near some bifurcation or critical points for flexible switching between coherent states, which, more generally, has been argued to bring about optimal computational capabilities.
We recently proposed that this dynamical system framework might also be useful in identifying the neuronal underpinnings of cognitive deficits in the course of extended wakefulness. In several studies involving human EEG and ECoG as well as rodents, we could show that the signatures of critical dynamics progressively fade during sustained wakefulness. These results point to a network-level function of sleep: to reorganize cortical networks towards a critical state to ensure optimal function for the time awake.
Fading signatures of critical brain dynamics during sustained wakefulness in humans
C. Meisel, E. Olbrich, O. Shriki, P. Achermann
Journal of Neuroscience 33(44), 17363-17372 (2013)
The interplay between long- and short-range temporal correlations shapes cortex dynamics across vigilance states
C. Meisel, A. Klaus, V. Vyazovskiy, D. Plenz
Journal of Neuroscience (2017)
Decline of long-range temporal correlations during sustained wakefulness in the human brain
C. Meisel, K. Bailey, P. Achermann, D. Plenz
Scientific Reports (2017)
Critical Slowing Down in Neural Systems
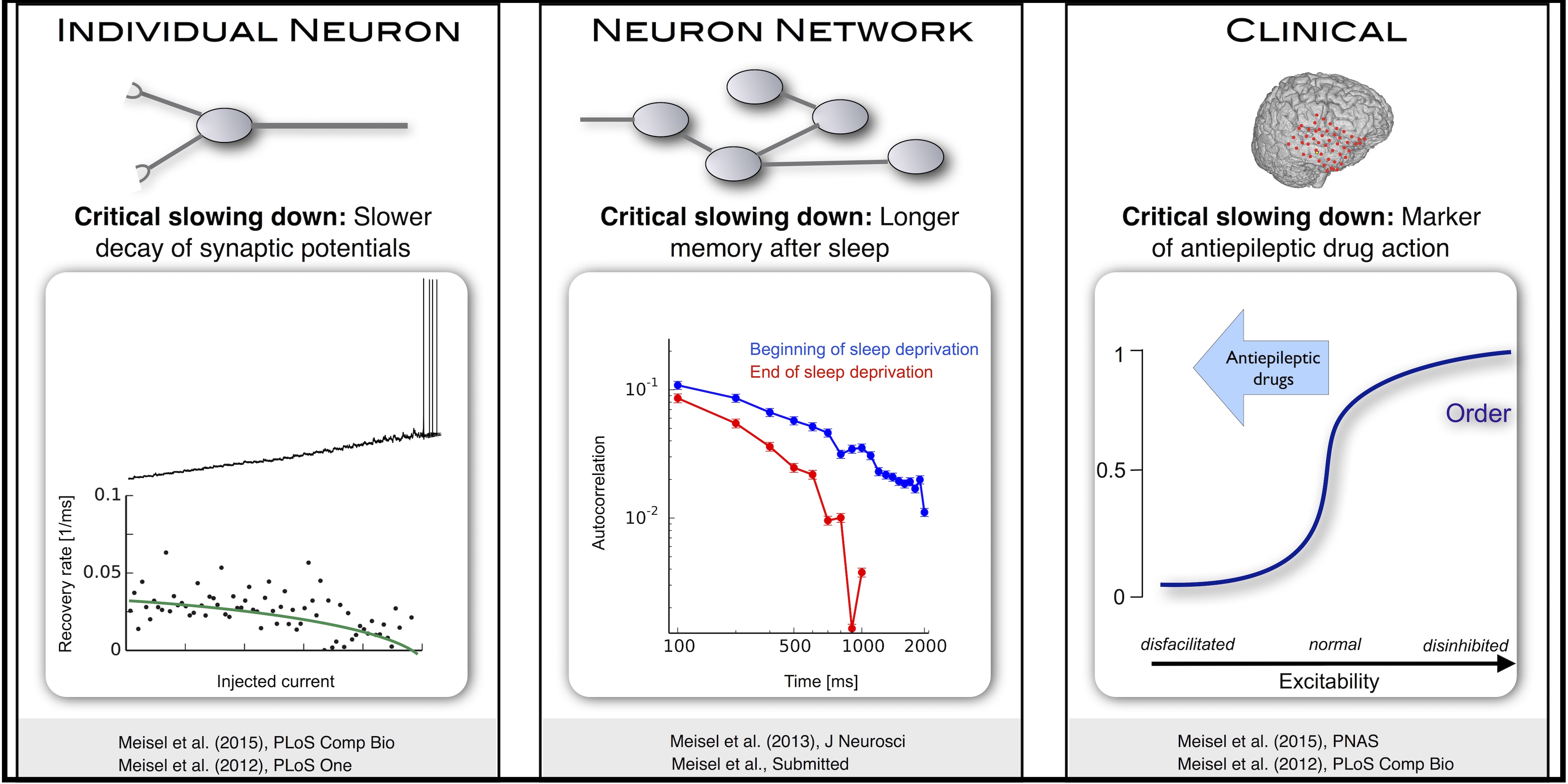
Many complex systems have been found to exhibit critical transitions, or so-called tipping points, which are sudden changes to a qualitatively different system state. These changes can profoundly impact the functioning of a system ranging from controlled state switching to a catastrophic break-down; signals that predict critical transitions are therefore highly desirable. To this end, research efforts have focused on utilizing qualitative changes in markers related to a system’s tendency to recover more slowly from a perturbation the closer it gets to the transition — a phenomenon called critical slowing down. The recently studied scaling of critical slowing down offers a refined path to understand critical transitions: to identify the transition mechanism and improve transition prediction using scaling laws.
We recently outlined and applied this strategy for the first time in a real-world system by studying the transition to spiking in neurons of the mammalian cortex as well as the onset of epileptic seizures in model system. We could show that these scaling laws allow for the identification of the underlying bifurcation and that they can be used to predict the bifurcation point from a limited window of observation. As such, the scaling laws present a missing link for a broad class of neuroscience modeling and suggest improved estimation of tipping points by incorporating scaling laws of critical slowing down as a strategy applicable to other complex systems.
Critical slowing governs the transition to neuronal spiking
C. Meisel, A. Klaus, C. Kuehn and D. Plenz (2015)
PLoS Comput. Biol. 11(2), e1004097
Scaling effects and spatio-temporal multilevel dynamics in epileptic seizures
C. Meisel and C. Kuehn (2012)
PLoS ONE 7(2), e30371